All about natural gas: composition and properties, production and use of natural gas
Due to its high energy efficiency and environmental friendliness, natural gas, along with oil, is of paramount importance.It is widely used as a fuel and also serves as a valuable raw material for the chemical industry.
And although the use of gas has become everyday and familiar, it still remains a complex and rather dangerous substance - in order to get into the burner of a gas appliance, it goes through a long and complex path.
In the article we will analyze the main issues related to natural flammable gas - we will talk about its composition and properties, we will describe the stages of gas production, transportation and processing, and the scope of its application. Let's consider modern ideas about the origin of hydrocarbon reserves, interesting facts and hypotheses.
The content of the article:
What is natural flammable gas?
There is an opinion that gas lies underground in voids and is easily extracted from there, for which it is enough to drill a well. But in reality, everything is much more complicated: gas can be inside a porous rock, it can be dissolved in water, liquid hydrocarbons, and oil.
To understand why this happens, it is enough to remember that the word “gas” comes from the Greek “chaos", which reflects the principle of behavior of a substance. In the gaseous state, molecules move chaotically, trying to uniformly fill the entire possible volume. Due to this, they are able to penetrate and dissolve in other substances, including denser liquids and minerals. High pressure and temperature significantly enhance the diffusion process.Often it is in the form of such a “cocktail” that natural gas is contained in the subsoil.
But first, let's talk about what the gas consists of and what it is - let's look at the chemical composition and physical properties of natural combustible gas.
Features of the chemical composition
Gas extracted from the subsoil, which is called “natural”, is a mixture of different gases.
According to its composition, it is divided into three groups of components:
- flammable – hydrocarbons;
- non-flammable (ballasts) – nitrogen, carbon dioxide, oxygen, helium, water vapor;
- harmful impurities – hydrogen sulfide and mercaptans.
The first and main group is a set of methane hydrocarbons (homologs) with the number of carbon atoms from 1 to 5. The largest percentage in the mixture is methane (from 70 to 98%), which has one carbon atom. The content of other gases (ethane, propane, butane, pentane) ranges from units to tenths of a percent.
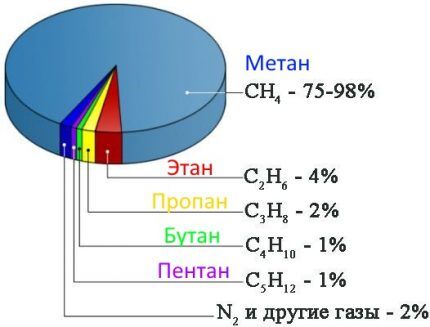
In addition to hydrocarbons, the mixture may contain non-flammable substances in small quantities: hydrogen sulfide, nitrogen, carbon dioxide, carbon monoxide, hydrogen and others. But, depending on the field, the proportions of hydrocarbons, like the composition of other gases, can vary significantly.
Physical properties of gas
According to the physical properties of methane CH4 colorless and odorless, very flammable. At air concentrations of more than 4.5% – explosive. This property, combined with the lack of odor, poses a great threat and problem. Especially in mines, since methane is absorbed by coal.
We wrote about the causes of gas explosions in domestic conditions in this material.
To give the gas an odor in order to detect its leaks, special substances with an unpleasant odor - odorants - are added to it before transportation. Most often, these are sulfur-containing compounds - ethanethiol or ethyl mercaptan. The proportion of the impurity is selected so that a leak is noticeable at a gas concentration of 1%.
Where does gas come from in the depths of the earth?
Although people learned to use gas more than 200 years ago, there is still no consensus on where gas comes from in the bowels of the earth.
Main theories of origin
There are two main theories of its origin:
- mineral, which explains the formation of gas by the processes of degassing of hydrocarbons from deeper and denser layers of the earth and their rise to zones with lower pressure;
- organic (biogenic), according to which gas is a product of the decomposition of the remains of living organisms under conditions of high pressure, temperature and lack of air.
In a field, gas can be in the form of a separate accumulation, a gas cap, a solution in oil or water, or gas hydrates. In the latter case, the deposits are located in porous rocks between gas-tight layers of clay. Most often, such rocks are compacted sandstone, carbonates, and limestones.
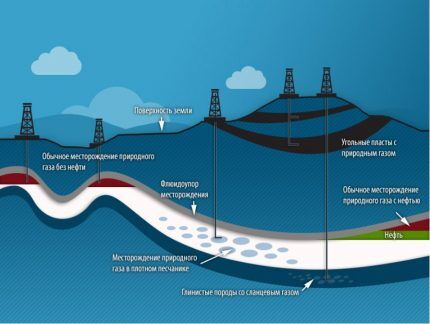
Since gas is lighter than oil, and water is heavier, the position of the minerals in the reservoir is always the same: gas is on top of the oil, and water props up the entire oil and gas field from below.
The gas in the formation is under pressure. The deeper the deposits, the higher it is. On average, for every 10 meters, the pressure increase is 0.1 MPa. There are layers with abnormally high pressure. For example, in the Achimov deposits of the Urengoy field it reaches 600 atmospheres and higher at a depth of 3800 to 4500 m.
Interesting facts and hypotheses
Not so long ago, it was believed that the world's oil and gas reserves should be exhausted at the beginning of the 21st century. For example, the authoritative American geophysicist Hubbert wrote about this in 1965.
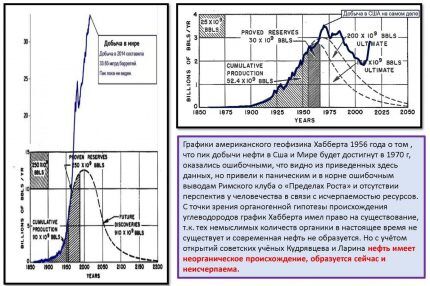
According to Doctor of Geological and Mineralogical Sciences V.V. Polevanova, such misconceptions are caused by the fact that the theory of the organic origin of oil and gas is still generally accepted and dominates the minds of most scientists. Although still D.I. Mendeleev substantiated the theory of the inorganic deep origin of oil, and then this was proven by Kudryavtsev and V.R. Larin.
But many facts speak against the organic origin of hydrocarbons.
Here are some of them:
- deposits have been discovered at depths of up to 11 km, in crystalline basements, where the existence of organic matter cannot even be theoretically possible;
- with the help of organic theory, only 10% of hydrocarbon reserves can be explained, the remaining 90% are inexplicable;
- The Cassini space probe discovered in 2000 on Saturn's moon Titan gigantic hydrocarbon resources in the form of lakes, several orders of magnitude larger than those on Earth.
The hypothesis of an initially hydrid Earth put forward by Larin explains the origin of hydrocarbons through the reaction of hydrogen with carbon in the depths of the earth and the subsequent degassing of methane.
According to it, there are no ancient deposits of the Jurassic period. All oil and gas could have formed between 1 and 15 thousand years ago. As selection proceeds, reserves can gradually be replenished, which has been noticed in long-depleted and abandoned oil fields.
How is extraction and transportation carried out?
The process of extracting natural combustible gas begins with the construction of wells. Depending on the occurrence of the gas-bearing formation, their depth can reach 7 km. As drilling progresses, a pipe (casing) is lowered into the well. To prevent gas from escaping through the space between the pipe and the walls of the well, plugging is done - filling the gap with clay or cement.
At the end of construction, the derrick is removed and the Xmas tree is installed on the casing head. It is a structure of gate valves and is used to extract gas from the well.
The number of wells can be quite large.

The entire cycle of natural combustible gas production occurs in three stages:
- Gas field development. As a result of drilling, a pressure difference is created. Due to this, gas moves through the formation to the wells.
- Operation of gas wells. At this stage, the gas travels through the casing.
- Collection and preparation for transportation. Gas from all Christmas trees is supplied to special technological complexes of the gas treatment plant. They dry the gas, cleaning from harmful impurities.
Even small concentrations of hydrogen sulfide, water vapor or solid particles lead to rapid corrosion, hydrate formation and mechanical damage to the internal surface of the pipeline.
Final preparation for transportation takes place at the headworks. It includes post-treatment and removal of hydrocarbon condensate, gas cooling to reduce its volume.
The main type of gas transportation over long distances is main gas pipeline. It is a system of complex engineering structures from the pipelines themselves to underground storage facilities.
At the final point of the pipeline there are gas distribution stations (GDS). Here the final cleaning of dust and liquid impurities takes place, the pressure is reduced to the level required by consumers, it is stabilized, gas consumption is taken into account and an odorant is added.
Another common type of methane transportation is sea transportation by special vessels - gas carriers.

The transformation of gas into a liquid state is carried out at special LNG plants. The process occurs in two stages: first, the methane is cooled to -50 °C, and then to -163 °C. At the same time, its volume decreases by 600 times.
Processing and scope of application
The high flammability of natural gas determines its main use. It is used as fuel in factories, factories, thermal power plants, boiler houses, institutions, residential buildings, agricultural facilities and many others. We recommend that you read the rules use of gas in everyday life.
Oil production and refining is always accompanied by the release of associated gas. In some cases, its volumes can be impressive and amount to up to 300 cubic meters per cubic meter of crude oil.
But there are a large number of fields where natural associated gas is not used, but is flared. For example, throughout Russia, up to 25% of useful raw materials are lost in this way.
Part of the associated gas is supplied to gas processing plants. From it, purified dry gas is obtained, which is used for heating. Another valuable component is a mixture of light hydrocarbons.
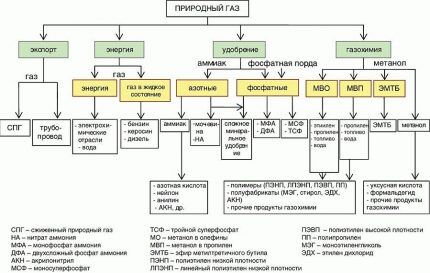
It is then divided into fractions in special installations. The result is hydrocarbons such as propane, butane, isobutane, and pentane. To reduce volume, ease of transportation and storage of them liquefy.

Propane and butane are used for heating homes bottled gas or for cars. But most of it goes for further processing in petrochemical production.
By high-temperature heating (pyrolysis), they are used to obtain the main raw material for all synthetic materials - monomers: ethylene, propylene, butadiene. Under the influence of catalysts they are combined into polymers. The output is such valuable materials as rubber, PVC, polyethylene and many others.
Conclusions and useful video on the topic
The documentary tells about gas in an accessible and visual way:
This educational film is dedicated to the main gas transport:
We still don’t know everything about natural gas—its origin still holds many mysteries. We can only hope that blue fuel is truly an inexhaustible gift that will be enough for us and our descendants.
Do you still have questions after reading the material above? Or do you want to supplement the article with useful comments, interesting facts or photographs? Write your comments, ask questions, participate in the discussion - the feedback form is located below.



