Amine gas purification from hydrogen sulfide: principle, effective options and plant diagrams
Natural gas extracted from fields for delivery to consumers via pipelines contains sulfur compounds in different proportions.If you do not get rid of them, aggressive substances will destroy the pipeline and render the fittings unusable. In addition, when contaminated blue fuel is burned, toxins are released.
In order to avoid negative consequences, amine gas purification from hydrogen sulfide is performed. This is the simplest and most inexpensive way to separate harmful components from fossil fuels. We will tell you how the process of separation of sulfur inclusions occurs, how the purification plant is designed and operates.
The content of the article:
Purpose of fossil fuel cleanup
Gas is the most popular type of fuel. It attracts with the most affordable price and causing the least damage to the environmental situation. The undeniable advantages include the ease of controlling the combustion process and the ability to secure all stages of fuel processing during the production of thermal energy.
However, the natural gaseous mineral is not mined in its pure form, because Simultaneously with gas extraction, associated organic compounds are pumped out from the well. The most common of them is hydrogen sulfide, the content of which varies from tenths to ten percent or more, depending on the deposit.
Hydrogen sulfide is poisonous, hazardous to the environment, and harmful to catalysts used in gas processing. As we have already noted, this organic compound is extremely aggressive towards steel pipes and metal valves.
Naturally, corroding the private system and main gas pipeline, hydrogen sulfide leads to leaks of blue fuel and extremely negative, risky situations associated with this fact. To protect the consumer, compounds harmful to health are removed from the gaseous fuel before it is delivered to the pipeline.
According to the standards, hydrogen sulfide compounds in gas transported through pipes cannot exceed 0.02 g/m³. However, in fact there are much more of them. In order to achieve the value regulated by GOST 5542-2014, cleaning is required.
Existing methods for hydrogen sulfide separation
In addition to hydrogen sulfide, which predominates among other impurities, blue fuel may also contain other harmful compounds. Carbon dioxide, light mercaptans and carbon sulfide can be found in it. But hydrogen sulfide itself will always predominate.
It is worth noting that some minor content of sulfur compounds in purified gaseous fuel is acceptable. The specific tolerance figure depends on the purposes for which the gas is produced.For example, to produce ethylene oxide, the total content of sulfur impurities must be less than 0.0001 mg/m³.
The cleaning method is chosen based on the desired result.
All currently existing methods are divided into two groups:
- Sorptive. They involve the absorption of hydrogen sulfide compounds by a solid (adsorption) or liquid (absorption) reagent with the subsequent release of sulfur or its derivatives. After which the harmful impurities separated from the gas are disposed of or processed.
- Catalytic. They consist of the oxidation or reduction of hydrogen sulfide, converting it into elemental sulfur. The process is carried out in the presence of catalysts - substances that stimulate the course of a chemical reaction.
Adsorption involves collecting hydrogen sulfide by concentrating it on the surface of a solid. Most often, granular materials based on activated carbon or iron oxide are used in the adsorption process. The large specific surface area characteristic of grains contributes to maximum retention of sulfur molecules.
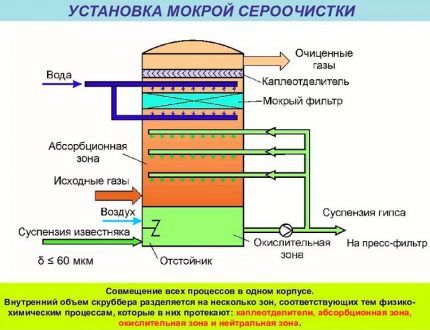
Absorption technology differs in that gaseous hydrogen sulfide impurities are dissolved in the active liquid substance. As a result, gaseous pollutants pass into the liquid phase. Then the isolated harmful components are removed by stripping, otherwise desorption, by this method they are eliminated from the reactive liquid.
Despite the fact that adsorption technology refers to “dry processes” and allows for fine purification of blue fuel, absorption is more often used to remove contaminants from natural gas. Collection and elimination of hydrogen sulfide compounds using liquid absorbents is more profitable and expedient.

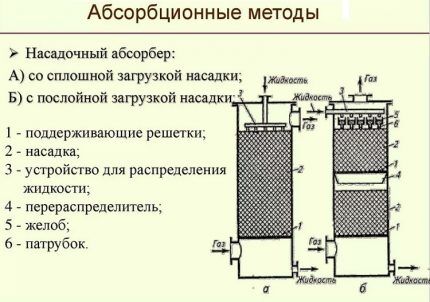
Absorption methods used in gas purification are divided into the following three groups:
- Chemical. Manufactured using solvents that readily react with hydrogen sulfide acidic pollutants. Ethanolamines or alkanolamines have the highest absorption capacity among chemical sorbents.
- Physical. They are performed by physically dissolving hydrogen sulfide gas in a liquid absorber. Moreover, the higher the partial pressure of the gaseous pollutant, the faster the dissolution process occurs. Methanol, propylene carbonate, etc. are used as absorbers here.
- Combined. In the mixed version of hydrogen sulfide extraction, both technologies are involved. The main work is done by absorption, and fine purification is carried out by adsorbents.
For half a century, the most popular and popular technology for separating and removing hydrogen sulfide and carbonic acid from natural fuels has been chemical gas purification using an amine sorbent used in the form of an aqueous solution.
Amine technology is more suitable for processing large volumes of gas because:
- No shortage. Reagents can always be purchased in the volume required for cleaning.
- Acceptable absorption. Amines are characterized by high absorption capacity. Of all the substances used, only they are capable of removing 99.9% of hydrogen sulfide from gas.
- Priority characteristics. Aqueous amine solutions are characterized by the most acceptable viscosity, vapor density, thermal and chemical stability, and low heat capacity. Their characteristics ensure the best course of the absorption process.
- No toxicity of reactive substances. This is an important argument that convinces one to resort to the amine method.
- Selectivity. Quality required for selective absorption. It provides the ability to sequentially carry out the necessary reactions in the order required for the optimal result.
Ethanolamines used in chemical methods for purifying gas from hydrogen sulfide and carbon dioxide include monoethanolamines (MEA), diethanolamines (DEA), and triethanolamines (TEA). Moreover, substances with the prefixes mono- and di- are removed from the gas and H2S, and CO2. But the third option helps to remove only hydrogen sulfide.
When performing selective cleaning of blue fuel, methyldiethanolamines (MDEA), diglycolamines (DGA), and diisopropanolamines (DIPA) are used. Selective absorbents are mainly used abroad.
Naturally, ideal absorbents that satisfy all cleaning requirements before delivery to the system gas heating and supply of other equipment does not yet exist. Each solvent has some advantages along with disadvantages. When choosing a reactive substance, simply determine the most suitable one from a number of proposed ones.
Operating principle of a typical installation
Maximum absorption capacity in relation to H2S is characterized by a solution of monoethanolamine. However, this reagent has a couple of significant drawbacks. It is characterized by fairly high pressure and the ability to create irreversible compounds with carbon sulphide during operation of the amine gas purification unit.
The first disadvantage is eliminated by washing, as a result of which the amine vapors are partially absorbed. The second one is rarely encountered during the processing of field gases.
The concentration of an aqueous solution of monoethanolamine is selected experimentally, and based on the research carried out, it is used to purify gas from a specific field. The selection of the percentage of the reagent takes into account its ability to withstand the aggressive effects of hydrogen sulfide on the metal components of the system.
Typical absorbent contents are typically in the range of 15 to 20%. However, it often happens that the concentration is increased to 30% or reduced to 10%, depending on how high the degree of purification should be. Those. for what purpose, in heating or in the production of polymer compounds, will the gas be used.
Note that with increasing concentration of amine compounds, the corrosive potential of hydrogen sulfide decreases. But we must take into account that in this case the consumption of the reagent increases. Consequently, the cost of purified commercial gas increases.
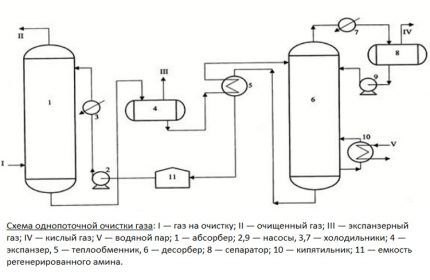
The main unit of the purification plant is an absorber of the plate or mounted variety. This is a vertically oriented apparatus, resembling a test tube in appearance, with nozzles or plates located inside. At its lower part there is an inlet for supplying an unpurified gas mixture, at the top there is an outlet to the scrubber.
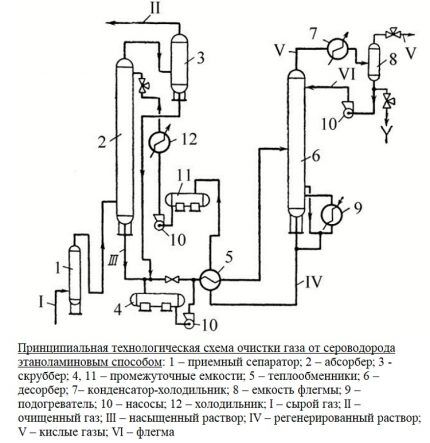
The gas flow, after passing through the inlet separator, is forced into the lower section of the absorber. It then passes through plates or nozzles located in the middle of the body, on which contaminants are deposited. The nozzles, completely wetted with the amine solution, are separated from each other by grids for uniform distribution of the reagent.
Next, the blue fuel, cleared of contaminants, is sent to the scrubber. This device can be connected in the processing circuit after the absorber or located in its upper part.
The spent solution flows down the walls of the absorber and is sent to a stripping column - a stripper with a boiler. There, the solution is cleaned of absorbed contaminants by vapors released when water is boiled in order to be returned back to the installation.
Regenerated, i.e. freed from hydrogen sulfide compounds, the solution flows into the heat exchanger. In it, the liquid is cooled in the process of transferring heat to the next portion of the contaminated solution, after which it is pumped into the refrigerator for complete cooling and condensation of the steam.
The cooled absorbent solution is fed back into the absorber. This is how the reagent circulates throughout the installation. Its vapors are also cooled and cleared of acidic impurities, after which they replenish the reagent supply.
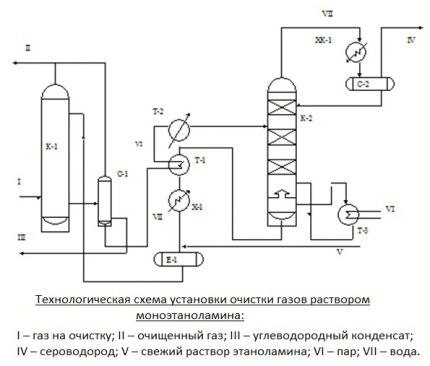
If it is necessary to simultaneously remove CO from the gas being processed2 and H2S, two-stage cleaning is performed.It consists of using two solutions that differ in concentration. This option is more economical than one-step cleaning.
First, gaseous fuel is cleaned with a strong composition containing a reagent of 25-35%. Then the gas is treated with a weak aqueous solution, in which the active substance is only 5-12%. As a result, both coarse and fine cleaning are performed with minimal solution consumption and reasonable use of the generated heat.
Four alconolamine cleaning options
Alconolamines or amino alcohols are substances containing not only an amine group, but also a hydroxy group.
The design of installations and technologies for purifying natural gas with alkanolamines differ mainly in the method of supplying the absorbent substance. Most often, four main methods are used in gas cleaning using this type of amines.
First way. Predetermines the supply of the active solution in one stream from above. The entire volume of absorbent is directed to the upper plate of the installation. The cleaning process occurs at a temperature background of no higher than 40ºС.
This technique is usually used for minor contamination with hydrogen sulfide compounds and carbon dioxide. The total thermal effect for producing commercial gas is, as a rule, low.
Second way. This cleaning option is used when there is a high content of hydrogen sulfide compounds in gaseous fuel.
In this case, the reagent solution is supplied in two streams. The first, with a volume of approximately 65-75% of the total mass, is sent to the middle of the installation, the second is supplied from above.
The amine solution flows down the trays and meets the rising gas flows, which are forced onto the lower tray of the absorbent unit. Before supply, the solution is heated to no more than 40ºC, but during the interaction of the gas with the amine, the temperature rises significantly.
To prevent the cleaning efficiency from decreasing due to an increase in temperature, excess heat is removed along with the waste solution saturated with hydrogen sulfide. And at the top of the installation, the flow is cooled in order to extract the remaining acidic components along with condensate.
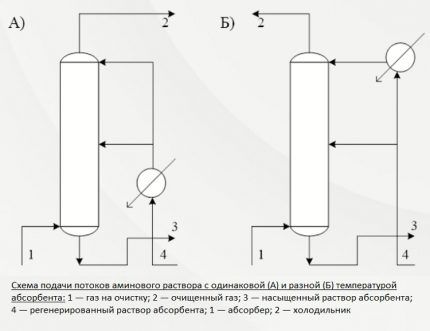
This is an economical method that reduces the consumption of both energy and active solution. Additional heating is not performed at any stage. In its technological essence, it is a two-level purification, which provides the opportunity to prepare commercial gas for supply to the pipeline with minimal losses.
Third way. It involves supplying the absorber to the cleaning installation in two streams of different temperatures. The method is used if, in addition to hydrogen sulfide and carbon dioxide, the raw gas also contains CS2, and COS.
The predominant part of the absorber, approximately 70-75%, heats up to 60-70ºС, and the remaining portion only to 40ºС. Flows are fed into the absorber in the same way as in the case described above: from above and into the middle.
The formation of a high-temperature zone makes it possible to quickly and efficiently remove organic contaminants from the gas mass at the bottom of the cleaning column. And at the top, carbon dioxide and hydrogen sulfide are precipitated by an amine at standard temperature.
Fourth method. This technology predetermines the supply of an aqueous amine solution in two streams with different degrees of regeneration. That is, one is supplied in an unrefined form, containing hydrogen sulfide inclusions, the second - without them.
The first stream cannot be called completely polluted. It only partially contains acidic components, because some of them are removed during cooling to +50º/+60ºC in the heat exchanger. This solution flow is taken from the bottom stripper nozzle, cooled and directed to the middle part of the column.
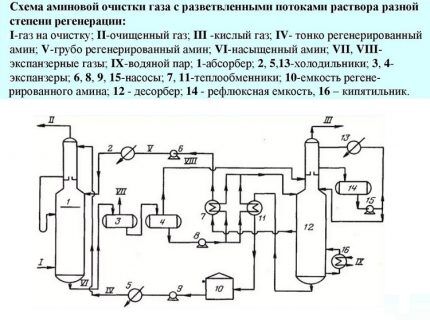
Only that part of the solution that is pumped into the upper sector of the installation undergoes deep cleaning. The temperature of this stream usually does not exceed 50ºС. Here, fine cleaning of gaseous fuel is performed. This scheme allows you to reduce costs by at least 10% by reducing steam consumption.
It is clear that the cleaning method is chosen based on the presence of organic contaminants and economic feasibility. In any case, the variety of technologies allows you to choose the best option. At the same amine gas treatment plant, it is possible to vary the degree of purification, obtaining blue fuel with the required for work gas boilers, stoves, heaters characteristics.
Conclusions and useful video on the topic
The following video will introduce you to the specifics of extracting hydrogen sulfide from associated gas produced along with oil by an oil well:
The video will present the installation for purifying blue fuel from hydrogen sulfide to produce elemental sulfur for further processing:
The author of this video will tell you how to get rid of hydrogen sulfide from biogas at home:
The choice of gas purification method is, first of all, focused on solving a specific problem. The performer has two options: follow a proven scheme or prefer something new. However, the main guideline should still be economic feasibility while maintaining quality and obtaining the required degree of processing.



