How and why gas is liquefied: production technology and scope of use of liquefied gas
Technologies related to the production, transportation and processing of natural gas are developing at a rapid pace.And many people today hear the abbreviations LPG and LNG. Almost every other day, natural gas fuel is mentioned in the news in one context or another.
But, you see, in order to have a clear understanding of what is happening, it is important to initially understand how gas is liquefied, why it is done, and what benefits it does or does not provide. And there are a lot of nuances in this issue.
To liquefy gaseous hydrocarbons, large high-tech plants are being built. Next, we will carefully look at why all this is needed and how it happens.
The content of the article:
Why is natural gas liquefied?
Blue fuel is extracted from the bowels of the earth in the form of a mixture of methane, ethane, propane, butane, helium, nitrogen, hydrogen sulfide and other gases, as well as their various derivatives.
Some of them are used in the chemical industry, and some are burned in boilers or turbines to generate thermal and electrical energy. Plus, some of the extracted volume is used as gas engine fuel.

The main reason for liquefying natural gas is to simplify its transportation over long distances. If the consumer and the gas fuel production well are located on land not far from each other, then it is easier and more profitable to lay a pipe between them.But in some cases, building a highway is too expensive and problematic due to geographical nuances. Therefore, they resort to various technologies for producing LNG or LPG in liquid form.
Economics and safety of transportation
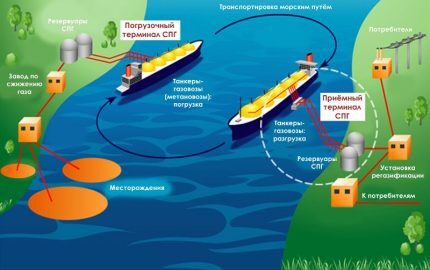
After the gas is liquefied, it is pumped into liquid form into special containers for transportation by sea, river, road and/or rail. At the same time, technologically, liquefaction is a fairly costly process from an energy point of view.
At different plants, this takes up to 25% of the original volume of fuel. That is, to generate the energy required by the technology, you have to burn up to 1 ton of LNG for every three tons of it in finished form. But natural gas is now in great demand, everything is paying off.
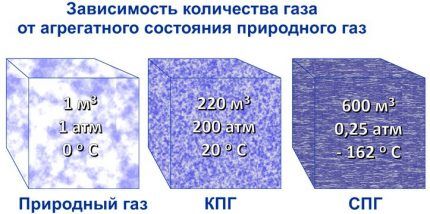
While natural gas is a liquid, it is non-flammable and non-explosive. Only after evaporation during regasification, the resulting gas mixture turns out to be suitable for burning in boilers and cookers. Therefore, if LNG or LPG is used as hydrocarbon fuel, then they must be regasified.
Use in various fields
Most often, the terms “liquefied gas” and “gas liquefaction” are mentioned in the context of transportation of hydrocarbon energy carriers. That is, first, blue fuel is extracted, and then it is converted into LPG or LNG. The resulting liquid is then transported and then returned to a gaseous state for one use or another.

LPG from propane-butane is mainly used as:
- gas engine fuel;
- fuel for pumping into gas tanks of autonomous heating systems;
- liquids for refilling lighters and gas cylinders with a capacity from 200 ml to 50 l.
LNG is typically produced exclusively for long-distance transportation. If a container capable of withstanding pressure of several atmospheres is sufficient for storing LPG, then special cryogenic tanks are required for liquefied methane.
LNG storage equipment is highly technological and takes up a lot of space. It is not profitable to use such fuel in passenger cars due to the high cost of cylinders. LNG-powered trucks in the form of single experimental models are already driving on the roads, but in the passenger car segment this “liquid” fuel is unlikely to find widespread use in the near future.
Liquefied methane as a fuel is now increasingly used in operation:
- railway diesel locomotives;
- sea vessels;
- river transport.
In addition to being used as an energy carrier, LPG and LNG are also used directly in liquid form in gas and petrochemical plants. They are used to make various plastics and other hydrocarbon-based materials.
Technologies for obtaining LPG and LNG
To convert methane from gas to liquid, it must be cooled to -163 °C. And propane-butane liquefies at -40 °C. Accordingly, technologies and costs in both cases are very different.
The following technologies from different companies are used to liquefy natural gas:
- AP-SMR (AP-X, AP-C3MR);
- Optimized Cascade;
- DMR;
- PRICO;
- MFC;
- GTL et al.
All of them are based on compression and/or heat exchange processes. The liquefaction operation takes place at the plant in several stages, during which the gas is gradually compressed and cooled to the temperature of transition into the liquid phase.
Preparation of the gas mixture
Before you can liquefy raw natural gas, you must remove water, helium, hydrogen, nitrogen, sulfur compounds and other impurities from it. For this purpose, adsorption technology is usually used for deep purification of the gas mixture by passing it through molecular sieves.
Then the second stage of feedstock preparation occurs, during which heavy hydrocarbons are removed. As a result, only ethane and methane (or propane and butane) with a volume of impurities of less than 5% remain in the gas, so that this fraction can begin to be cooled and liquefied.
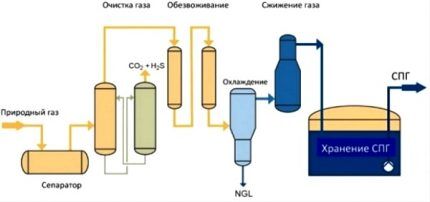
Fractionation allows you to get rid of harmful impurities and isolate only the main gas for subsequent liquefaction. At a pressure of 1 atm, the temperature of transition into the liquid state for methane is -163 °C, for ethane -88 °C, for propane -42 °C, and for butane -0.5 °C.
It is precisely these temperature differences that explain the reason why the gas entering the plant is divided into fractions and only then liquefied. There is no single liquefaction technology for all types of gaseous hydrocarbon compounds. For each of them it is necessary to build and use its own production line.
Basic liquefaction process
The basis for converting gas into a liquid state is the refrigeration cycle, during which heat is transferred by one or another refrigerant from an environment with a low temperature to an environment with a higher one. This process is multi-stage and requires powerful compressors for expansion/compression of the coolant and heat exchangers.
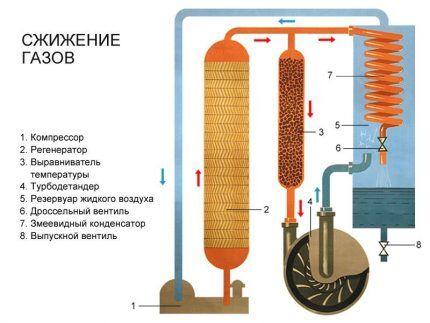
The following are used as a refrigerant at different stages of liquefaction:
- propane;
- methane;
- ethane;
- nitrogen;
- water (sea and purified);
- air.
For example, for the primary cooling of natural gas at Novatek’s Yamal LNG, cool Arctic air is used, which allows the temperature of the feedstock to be lowered at minimal cost immediately to +10 °C. And in the hot summer months, it is instead provided for the use of sea water from the Arctic Ocean, which, regardless of the time of year, at a depth of constant 3–4 ° C.
At the same time, nitrogen, obtained directly on site from the air, is used as the final refrigerant in Yamal. As a result, the Arctic provides everything needed to produce LNG - from the initial natural gas to the working agents used in the liquefaction process.
Propane liquefies in a similar way to methane. Only it requires much lower cooling temperatures - minus 42 °C versus minus 163 °C. Therefore liquefaction gas for gas tanks It costs several times less, but the resulting propane-butane LPG itself is less in demand on the market.
Transportation and storage
Almost the entire volume of LNG is transported by large-sized sea gas tankers from one coast to another.Transportation by land is limited by the need to maintain the temperature of the “liquid blue fuel” at values of about -160 ° C, otherwise methane begins to transform into a gas state and becomes explosive.

The pressure in the LNG tank is close to atmospheric. However, if the temperature of liquid methane rises above -160 °C, it will begin to turn from a liquid into a gas. As a result, the pressure in the container will begin to increase, which poses a serious danger. Therefore, LNG tankers are equipped with low temperature maintenance units and a thick layer of heat insulation.
LPG is regasified into gas directly in the gas tank. And LNG regasification is carried out in special industrial installations without access to oxygen. According to physics, liquid methane at positive temperatures gradually turns into gas. However, if this happens directly in the air outside of special conditions, then such a process will lead to an explosion.
After natural gas in the form of LNG is liquefied at the plant, it is transported, and then again at the plant (regasification only) it is converted back into a gaseous state for further use.
Prospects for liquefied hydrogen
In addition to direct liquefaction and use in this form, it is also possible to obtain another energy carrier from natural gas - hydrogen. Methane is CH4, propane C3N8, and butane C4N10.
The hydrogen component is present in all these fossil fuels, you just need to isolate it.
To transform hydrogen from a gas into a liquid, it must be cooled to -253 °C. For this purpose, multi-stage cooling systems and “compression/expansion” installations are used. Currently, such technologies are too expensive, but work is underway to reduce their cost.
We also recommend reading our other article, where we described in detail how to make a hydrogen generator for your home with your own hands. More details - go link.
Also, unlike LPG and LNG, liquefied hydrogen is much more explosive. The slightest leak of it in combination with oxygen produces a gas-air mixture that ignites at the slightest spark. And storage of liquid hydrogen is possible only in special cryogenic containers. Hydrogen fuel still has too many disadvantages.
Conclusions and useful video on the topic
How is liquefied gas produced and why is it liquefied:
All about liquefied gases:
There are several technologies for liquefying gases. For methane they are theirs, and for propane-butane they are theirs. At the same time, it is cheaper to obtain LPG, and it is easier and safer to transport/store. Producing methane LNG is a more expensive and complex process. Plus, its regasification requires specialized equipment. At the same time, methane is in greater demand on the market today, so it is liquefied in much larger volumes.
Do you have any clarifying questions or your own expert opinion on the topic of gas liquefaction? Perhaps you have something to add to the above. Feel free to ask and/or comment on the article in the box below.



