Calorific value of various types of fuel: comparison of fuels by calorific value + calorific value table
When a certain amount of fuel is burned, a measurable amount of heat is released.According to the International System of Units, the value is expressed in Joules per kg or m3. But the parameters can also be calculated in kcal or kW. If the value is related to a unit of fuel measurement, it is called specific.
What affects the calorific value of various fuels? What is the value of the indicator for liquid, solid and gaseous substances? The answers to the above questions are described in detail in the article. In addition, we have prepared a table displaying the specific heat of combustion of materials - this information will be useful when choosing a high-energy type of fuel.
The content of the article:
General information about calorific value
The release of energy during combustion should be characterized by two parameters: high efficiency and the absence of production of harmful substances.
Artificial fuel is obtained by processing natural fuel - biological fuel. Regardless of the state of aggregation, substances in their chemical composition have a flammable and non-flammable part. The first is carbon and hydrogen. The second consists of water, mineral salts, nitrogen, oxygen, and metals.
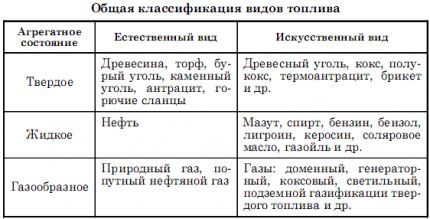
When 1 kg of such a “mixture” is burned, different amounts of energy are released. Exactly how much of this energy will be released depends on the proportions of these elements - the combustible part, humidity, ash content and other components.
The heat of combustion of fuel (HCT) is formed from two levels - the highest and the lowest. The first indicator is obtained due to water condensation; in the second, this factor is not taken into account.
The lowest TCT is needed to calculate the need for fuel and its cost; with the help of such indicators, heat balances are compiled and the efficiency of fuel-burning installations is determined.
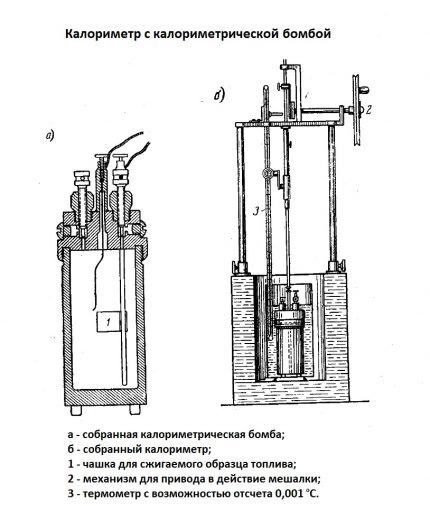
TST can be calculated analytically or experimentally. If the chemical composition of the fuel is known, the periodic formula is applied. Experimental techniques are based on the actual measurement of heat from fuel combustion.
In these cases, a special combustion bomb is used - a calorimetric one together with a calorimeter and a thermostat.
Features of the calculations are individual for each type of fuel. Example: TCT in internal combustion engines is calculated from the lowest value, because the liquid does not condense in the cylinders.
Each type of substance has its own TST due to the characteristics of its chemical composition. The values vary significantly, the range of fluctuations is 1,000–10,000 kCal/kg.
When comparing different types of materials, the concept of reference fuel is used; it is characterized by a lower TCT of 29 MJ/kg.
Calorific value of solid materials
This category includes wood, peat, coke, oil shale, briquette and pulverized fuel. The main component of solid fuel is carbon.
Features of different types of wood
Maximum efficiency from the use of firewood is achieved provided that two conditions are met - dry wood and a slow combustion process.

Ideal for wood stove heating oak, birch, ash bars are considered. Hawthorn and hazel are characterized by good indicators. But coniferous trees have a low calorific value, but a high burning rate.
How different rocks burn:
- Beech, birch, ash, hazel difficult to melt, but they are capable of burning moist due to their low moisture content.
- Alder with aspen do not form soot and “know how” to remove it from the chimney.
- Birch requires a sufficient amount of air in the firebox, otherwise it will smoke and deposit resin on the walls of the pipe.
- Pine contains more resin than spruce, so it sparks and burns hotter.
- Pear and apple tree It splits easier than others and burns well.
- Cedar gradually turns into smoldering coal.
- Cherry and elm it smokes, and the plane tree is difficult to split.
- Linden with poplar burn out quickly.
TST indicators of different breeds strongly depend on the density of specific breeds. 1 cubic meter of firewood is equivalent to approximately 200 liters of liquid fuel and 200 m3 natural gas. Wood and firewood fall into the low energy efficiency category.
Effect of age on coal properties
Coal is a natural material of plant origin. It is mined from sedimentary rocks. This fuel contains carbon and other chemical elements.
In addition to the type, the heat of combustion of coal is also influenced by the age of the material. Brown belongs to the young category, followed by stone, and anthracite is considered the oldest.

The process of coal combustion is accompanied by the release of substances that pollute the environment, and the boiler grates are covered with slag. Another unfavorable factor for the atmosphere is the presence of sulfur in the fuel. This element, upon contact with air, transforms into sulfuric acid.
Manufacturers manage to reduce the sulfur content in coal as much as possible. As a result, TST differs even within the same species. The production geography also influences the performance. Not only pure coal, but also briquetted slag can be used as solid fuel.
The highest fuel capacity is observed in coking coal. Stone, charcoal, brown coal, and anthracite also have good characteristics.
Characteristics of pellets and briquettes
This solid fuel is produced industrially from various wood and plant waste.
Shredded shavings, bark, cardboard, straw are dried and using special equipment turns into granules. In order for the mass to acquire a certain degree of viscosity, a polymer, lignin, is added to it.

Briquettes differ only in shape; they can be loaded into furnaces and boilers. Both types of fuel are divided into types based on raw materials: round timber, peat, sunflower, straw.
U pellets and briquettes there are significant advantages over other types of fuel:
- complete environmental friendliness;
- possibility of storage in almost any conditions;
- resistance to mechanical stress and fungus;
- uniform and long burning;
- optimal granule size for loading into a heating device.
Eco-friendly fuel is a good alternative to traditional heat sources, which are not renewable and have an adverse effect on the environment. But pellets and briquettes are characterized by an increased fire hazard, which should be taken into account when organizing a storage location.
If desired, you can arrange the production of fuel briquettes yourself; for more details, see this article.
Parameters of liquid substances
Liquid materials, like solid ones, are decomposed into the following components: carbon, hydrogen, sulfur, oxygen, nitrogen. The percentage is expressed by weight.
Internal organic ballast of fuel is formed from oxygen and nitrogen; these components do not burn and are included in the composition conditionally. External ballast is formed from moisture and ash.
Gasoline has a high specific heat of combustion. Depending on the brand, it is 43-44 MJ.
Similar indicators of the specific heat of combustion are determined for aviation kerosene - 42.9 MJ. Diesel fuel also falls into the category of leaders in terms of calorific value - 43.4-43.6 MJ.

Liquid rocket fuel and ethylene glycol are characterized by relatively low TCT values. Alcohol and acetone have the minimum specific heat of combustion. Their performance is significantly lower than that of traditional motor fuel.
Properties of gaseous fuels
Gaseous fuel consists of carbon monoxide, hydrogen, methane, ethane, propane, butane, ethylene, benzene, hydrogen sulfide and other components. These figures are expressed as a percentage by volume.

Natural gas also has high calorific values.
They are equal to 41-49 MJ per kg. But, for example, pure methane has a higher calorific value - 50 MJ per kg.
Comparative table of indicators
The table presents the values of the mass specific heat of combustion of liquid, solid, and gaseous fuels.
| Type of fuel | Unit change | Specific heat of combustion | ||
| MJ | kW | kcal | ||
| Firewood: oak, birch, ash, beech, hornbeam | kg | 15 | 4,2 | 2500 |
| Firewood: larch, pine, spruce | kg | 15,5 | 4,3 | 2500 |
| Brown coal | kg | 12,98 | 3,6 | 3100 |
| Coal | kg | 27,00 | 7,5 | 6450 |
| Charcoal | kg | 27,26 | 7,5 | 6510 |
| Anthracite | kg | 28,05 | 7,8 | 6700 |
| Wood pellets | kg | 17,17 | 4,7 | 4110 |
| Straw pellets | kg | 14,51 | 4,0 | 3465 |
| Sunflower pellets | kg | 18,09 | 5,0 | 4320 |
| Sawdust | kg | 8,37 | 2,3 | 2000 |
| Paper | kg | 16,62 | 4,6 | 3970 |
| Vine | kg | 14,00 | 3,9 | 3345 |
| Natural gas | m3 | 33,5 | 9,3 | 8000 |
| Liquefied gas | kg | 45,20 | 12,5 | 10800 |
| Petrol | kg | 44,00 | 12,2 | 10500 |
| Dis. fuel | kg | 43,12 | 11,9 | 10300 |
| Methane | m3 | 50,03 | 13,8 | 11950 |
| Hydrogen | m3 | 120 | 33,2 | 28700 |
| Kerosene | kg | 43.50 | 12 | 10400 |
| Fuel oil | kg | 40,61 | 11,2 | 9700 |
| Oil | kg | 44,00 | 12,2 | 10500 |
| Propane | m3 | 45,57 | 12,6 | 10885 |
| Ethylene | m3 | 48,02 | 13,3 | 11470 |
The table shows that hydrogen has the highest TST indicators of all substances, not just gaseous ones. It belongs to high-energy fuels.
The product of hydrogen combustion is ordinary water. The process does not emit furnace slag, ash, carbon dioxide and carbon dioxide, which makes the substance an environmentally friendly combustible. But it is explosive and has a low density, so this fuel is difficult to liquefy and transport.
Conclusions and useful video on the topic
About the calorific value of different types of wood. Comparison of indicators per m3 and kg.
TCT is the most important thermal and operational characteristic of a fuel. This indicator is used in various areas of human activity: heat engines, power plants, industry, home heating and cooking.
Calorific value values help to compare different types of fuel according to the degree of energy released, calculate the required mass of fuel, and save on costs.
Do you have anything to add or have questions about the calorific value of different types of fuel? You can leave comments on the publication and participate in discussions - the contact form is in the lower block.




Yes... maybe we will live to see hydrogen boilers become commonplace - a dream!
Of course, heating with main gas is the best option, but, unfortunately, in our huge country it is not available to everyone. And if you choose between coal and pellets, I choose pellets. Coal also emits a lot of harmful substances during the combustion process, and then the slag needs to be disposed of somewhere. And the whole country sprinkles it on the roads in winter, and then in the spring they breathe carcinogenic dust, and then they wonder why so many people get sick.
Ash from pellets can be used to fertilize a garden, or a lawn - whoever has what.
The best firewood comes from deciduous trees - oak, birch. Birch is the most versatile and popular firewood - it produces enough heat, burns evenly, without a lot of smoke. Oak produces the most heat when compared to trees growing in our country. Aspen is good for cleaning chimneys. I do not recommend heating with coniferous wood - because of the resins, they produce a lot of smoke.
I think it’s extremely unprofitable to heat only with wood now. The only place where this is applicable is a bathhouse. And if we take heating of a village house, then coal, no matter what anyone says, is still ahead of all types of fuel, with the exception of mains gas. Gas in cylinders, gas holder, firewood, pellets, briquettes - everything has disadvantages.Somewhere there is a high price, somewhere there is bureaucracy with obtaining a bunch of permits and passing inspections. And I don’t see any significant downsides to coal. Of course, someday gasification, not in words, but in deeds, will reach our villages and the relevance of coal will decrease, but this will not happen soon.
For gasoline, diesel fuel, oil, kerosene... data per KILOGRAM 😉
Yes, that's right, thanks! Corrected.
So in what units are the data on diesel fuel indicated - in kg or in liters?
In the table, kcal is energy, and kW is power. For example, 2500 kcal is 2.9075 kWh. Or am I wrong?
Energy is expressed in kWh. But boiler "experts" and "authors" often measure energy in kW. This error occurs in many articles. This can lead to confusion in calculations.
For methane, hydrogen, propane, ethylene, the data is also per kilogram
The specific heat of combustion of hydrogen in air is 120 MJ/kg, per 1 m3, respectively, 10.8 MJ/m3. Hydrogen is a very light gas, density 0.09 kg/m3, so its mass in 1 m3 is 7.6 times less than natural gas. All the comparison tables I looked at had the same error.